This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new electrolyte to make better lithium-ion batteries
From smartphones to electric cars, lithium ion batteries have changed the way we power our lives. And in the push towards net zero carbon emissions globally, they will be a vital part of decarbonizing transport networks and switching to renewable energy.
To make batteries with a higher energy density—which would mean that an electric car, for example, could cover more distance before it needed recharging—researchers at NTNU are investigating the use of different materials for the battery's key components.
Swapping silicon for graphite
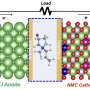
Batteries store energy by moving charged particles between two electrodes—a negatively charged anode and a positive charged cathode—through a liquid known as an electrolyte. In lithium-ion (Li-ion) batteries, lithium ions move from the cathode to the anode when the battery is charged, and are stored in the anode. When the battery is discharged, the ions move back to the cathode, producing an
Most existing Li-ion batteries use graphite for their anode. But swapping graphite for silicon could increase the energy density significantly.
"In lithium ion batteries, you would like to have anode and cathode materials that can store as much lithium as possible," says Ann Mari Svensson, a professor in NTNU's Department of Materials Science and Engineering. "Silicon can store huge amounts."
Combatting flammability
But Li-ion batteries have a reputation for being flammable. Switching out the electrolyte—the crucial component through which ions move between electrodes—is the main way to combat this.
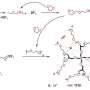
Svensson and colleagues are investigating the use of ionic liquids—essentially, salts that have such a low melting point that they are liquid at room temperature—to make batteries with a silicon electrode safer. "The current liquid electrolytes are partly to blame for thermal events," says Svensson. "If you can replace them with, for example, new ionic liquids, then the battery is less likely to catch fire."
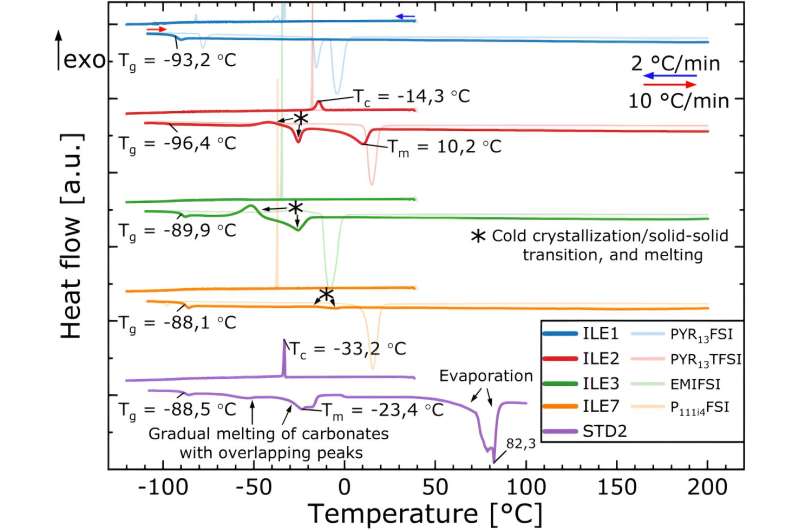
In work published in the Journal of The Electrochemical Society, Svensson and colleagues evaluated the performance of batteries with silicone anodes and ionic liquid electrolytes at high temperatures.
Increasing battery temperature helps
Increasing the temperature of the battery increases the conductivity of lithium ions within the electrolyte, overcoming the fact that ionic liquids don't tend to conduct lithium ions very well at room temperature. Demonstrating that these batteries can work at elevated temperatures could also make operating large packs of batteries, that have a tendency to overheat, simpler.
"Normally you have to cool the pack when it's operating. That could be, in theory, avoided," says Svensson.
During the course of their research, the team saw some surprising results. "We had studied these ionic liquids at room temperature before, but when we heated them to sixty degrees, then actually, the best one wasn't the best one anymore," says Svensson. "The ranking was quite different."
"One of them had a really excellent improvement in conductivity when going to the higher temperatures," she added.
Thin film on silicon is key
One driving factor behind some ionic liquid electrolytes performing better than others could lie in a key feature of Li-ion batteries with silicon electrodes: a thin film that forms over the silicon. When you start to charge a battery, lithium moves from the cathode to the anode. But in the process, the electrolyte starts to decompose and forms a thin film called a passivation layer on the surface of the anode.
"Once they're really covering the surface, the formation of these layers stops," says Svensson. "It's very important that these are intact, because otherwise you would just completely decompose most of your electrolyte."
For the ionic liquid electrolytes that performed well at the higher temperature, the researchers saw evidence of a stronger passivation layer as well as improved lithium ion mobility in the electrolyte.
More work before commercialization
Svensson and colleagues use something called X-ray photoelectron spectroscopy, or XPS, at NTNU's Nanolab, to investigate the films. "You need quite some advanced equipment to try to identify those films, they can be just a few nanometers thick," says Svensson.
After showing that ionic liquids can make a silicon anode a realistic option for Li-ion batteries, Svensson and her colleagues are now working on demonstrating how an ionic liquid electrolyte would work with a new type of cathode, too.
There are still many hurdles to overcome before these kinds of batteries could be available commercially. For one, ionic liquids are not currently mass produced in quantities that would be required for industrial production of batteries using them. And while research has shown promise when it comes to their short term performance, their long term performance—over the expected lifetime of a battery in the real world—remains to be seen.
"That's quite a big challenge," says Svensson.
More information: Daniel Tevik Rogstad et al, High-Temperature Performance of Selected Ionic Liquids as Electrolytes for Silicon Anodes in Li-ion Batteries, Journal of The Electrochemical Society (2022). DOI: 10.1149/1945-7111/ac9f78