Scientists identify another reason why batteries can't charge in minutes
Haste makes waste, as the saying goes. Such a maxim may be especially true of batteries, thanks to a new study that seeks to identify the reasons that cause the performance of fast charged lithium-ion batteries to degrade in electric vehicles.
In new research from the U.S. Department of Energy's (DOE) Argonne National Laboratory, scientists have found interesting chemical behavior of one of the battery's two terminals as the battery is charged and discharged.
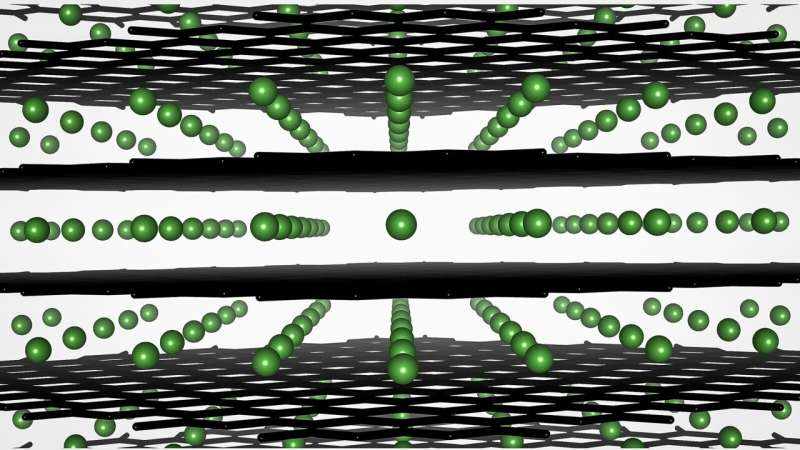
Lithium-ion batteries contain both a positively charged cathode and a negatively charged anode, which are separated by a material called an electrolyte that moves lithium ions between them. The anode in these batteries is typically made out of graphite—the same material found in many pencils. In lithium-ion batteries, however, the graphite is assembled out of small particles. Inside these particles, the lithium ions can insert themselves in a process called intercalation. When intercalation happens properly, the battery can successfully charge and discharge.
When a battery is charged too quickly, however, intercalation becomes a trickier business. Instead of smoothly getting into the graphite, the lithium ions tend to aggregate on top of the anode's surface, resulting in a "plating" effect that can cause terminal damage—no pun intended—to a battery.
"Plating is one of the main causes of impaired battery performance during fast charging," said Argonne battery scientist Daniel Abraham, an author of the study. "As we charged the battery quickly, we found that in addition to the plating on the anode surface there was a build up of reaction products inside the electrode pores." As a result, the anode itself undergoes some degree of irreversible expansion, impairing battery performance.
Using a technique called scanning electron nanodiffraction, Abraham and his colleagues from the University of Illinois Urbana-Champaign observed another notable change to the graphite particles. At the atomic level, the lattice of graphite atoms at the particle edges becomes distorted because of the repeated fast charging, hindering the intercalation process. "Basically, what we see is that the atomic network in the graphite becomes warped, and this prevents lithium ions from finding their 'home' inside the particles—instead, they plate on the particles," he said.
"The faster we charge our battery, the more atomically disordered the anode will become, which will ultimately prevent the lithium ions from being able to move back and forth," Abraham said. "The key is to find ways to either prevent this loss of organization or to somehow modify the graphite particles so that the lithium ions can intercalate more efficiently."
A paper based on the study, "Increased disorder at graphite particle edges revealed by multilength scale characterization of anodes from fast charged lithium-ion cells," appeared in the October 8 issue of the Journal of the Electrochemical Society.
More information: Saran Pidaparthy et al, Increased Disorder at Graphite Particle Edges Revealed by Multi-length Scale Characterization of Anodes from Fast-Charged Lithium-Ion Cells, Journal of The Electrochemical Society (2021). DOI: 10.1149/1945-7111/ac2a7f


















