This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Utilizing electron spin for faster charging, higher capacity batteries
Charging an electric vehicle usually takes about 10 hours or longer, and even with fast-charging methods, it takes at least 30 minutes. That is assuming there is an available spot at a charging station. If we could charge electric vehicles as quickly as refueling gasoline-powered cars, it could help alleviate the shortage of EV charging stations.
The efficiency of lithium-ion batteries, the type used in electric vehicles, is determined by the anode material's capability to store lithium ions. Recently, Professor Won Bae Kim, from the Department of Chemical Engineering and the Graduate Institute of Ferrous & Energy Materials Technology at Pohang University of Science and Technology (POSTECH, President Moo Hwan Kim), led a research team to develop a new anode material.
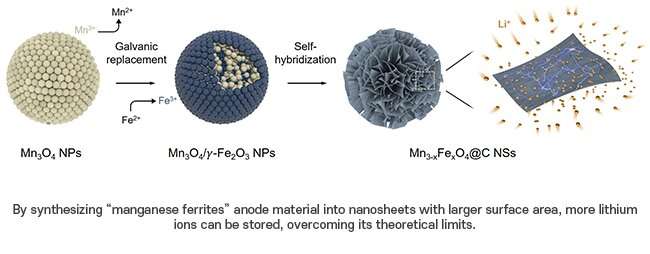
His team, which included Ph.D. candidates Song Kyu Kang and Minho Kim from the Department of Chemical Engineering, synthesized manganese ferrites (Mn3-xFexO4) nanosheets using a novel self-hybridization method involving a straightforward galvanic replacement-derived process.
This groundbreaking technique boosts storage capacity approximately 1.5 times above the theoretical limit and enables an electric vehicle to be charged in as little as six minutes. The research was recognized for its excellence and was published as a front cover paper in Advanced Functional Materials.
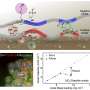
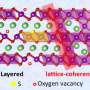
In this study, the research team devised a new method to synthesize manganese ferrites as anode material known for its superior lithium-ion storage capacity and ferromagnetic properties. First, a galvanic replacement reaction took place in a solution of manganese oxide mixed with iron, leading to a heterostructure compound with manganese oxide inside and iron oxide outside.
The team further used a hydrothermal method to create nanometer-thick sheets of manganese ferrites with expanded surface areas. This approach harnessed highly spin-polarized electrons, which significantly enhanced the storage capacity for a substantial quantity of lithium ions. This innovation allowed the team to effectively exceed the theoretical capacity of the manganese ferrites anode material by over 50 percent.
Enlarging the surface area of the anode material facilitated the simultaneous movement of a large quantity of lithium ions, thereby improving the battery's charging speed. Experimental results showed that just six minutes are required to charge and discharge a battery with a capacity equivalent to that used in EVs currently on the market. This study has refined the challenging synthesis process to make a breakthrough in the theoretical capacity of the anode material and significantly accelerate the battery charging process.
Professor Won Bae Kim, who spearheaded the research, stated, "We have offered a new understanding on how to overcome the electrochemical limitations of conventional anode materials and increase battery capacity by applying the rational design with surface alteration using electron spin." He expressed optimism that this development could lead to increased battery durability and reduced recharging time for electric vehicles.
More information: Song Kyu Kang et al, Exceeding Theoretical Capacity in Exfoliated Ultrathin Manganese Ferrite Nanosheets via Galvanic Replacement‐Derived Self‐Hybridization for Fast Rechargeable Lithium‐Ion Batteries, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202300143