This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop new green technology for producing hydrogen using renewable energy
A group of researchers from the Technion Faculty of Materials Science and Engineering has presented a new technology for producing green hydrogen using renewable energy. Their breakthrough was recently published in Nature Materials.
The novel technology embodies significant advantages compared to other processes for producing green hydrogen, and its development into a commercial technology is likely to reduce the costs and accelerate the use of green hydrogen as a clean, sustainable alternative to fossil fuels.
Using hydrogen as a fuel instead of coal, gasoline, and "natural" gas will reduce the use of these fuels and greenhouse gas emissions from various sources, including transportation, the production of materials and chemicals, and industrial heating. Unlike these fuels, which emit carbon dioxide into the atmosphere when they combust in the air, hydrogen produces water and is therefore considered a clean fuel.
However, the most common way to produce hydrogen involves using natural gas (or coal) and the process emits large amounts of carbon dioxide into the atmosphere—thereby canceling out its advantages as a green, sustainable alternative for fossil fuels. In 2022, global consumption of hydrogen stood at approximately 95 million tons—a quantity suitable for improving various fuel products, and especially to produce ammonia, which is needed for manufacturing agricultural fertilizers.
Nearly all of the hydrogen that is consumed today is produced from fossil fuels, which is why it is called "gray hydrogen" (made from methane) or "black hydrogen" (made from coal). Hydrogen production using these methods is responsible for around 2.5% of the annual global carbon dioxide emissions into the atmosphere as a result of human actions. Replacing gray hydrogen with green hydrogen is necessary in order to reduce this significant source of emissions and replace polluting fossil fuels with clean, sustainable hydrogen.
Various estimates predict that green hydrogen is likely to account for around 10% of the global energy market at net zero emissions—the current target for mitigating climate change and global warming as a result of the greenhouse effect due to increased concentration of carbon dioxide in the atmosphere. That is the reason for the enormous importance of green hydrogen in combating global warming.
Green hydrogen is produced through electrolysis—electrochemical decomposition of water into oxygen and hydrogen using energy from renewable sources such as wind and sun. Electrolysis was discovered more than 200 years ago, and since then it has undergone many developments and improvements. However, it is still too expensive for producing green hydrogen at a competitive price.
One of the technological challenges that limit the use of electrolysis for producing large amounts of green hydrogen—amounts that would help achieve plans to attain net zero carbon emissions—is the need for expensive membranes, gaskets and sealing components to separate the cathodic and anodic compartments.
Several years ago, Technion researchers presented an innovative and efficient electrolysis technique that doesn't require a membrane and sealing to separate the two parts of the cell, since the hydrogen and the oxygen are produced at different stages of the process, unlike in regular electrolysis where they are created simultaneously.
This novel process, called E-TAC, was developed by Dr. Hen Dotan and Dr. Avigail Landman under the supervision of Prof. Avner Rothschild and Prof. Gideon Grader. They partnered with the entrepreneur Talmon Marco to fulfill the process's potential and develop commercial applications.
Details of the new technology
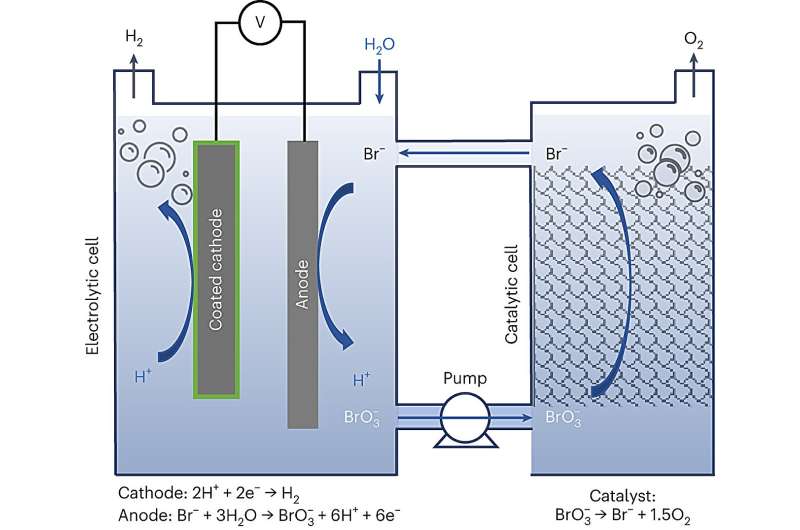
The researchers from Prof. Rothschild's group at the Technion now present a process whereby hydrogen and oxygen are produced simultaneously in two separate cells, unlike the E-TAC process where they are produced in the same cell but at different stages. The new process was developed by Ilia Slobodkin as part of his master's thesis, with the help of Senior Researcher Dr. Elena Davydova and Dr. Anna Breytus and master's student Matan Sananis.
This novel process bypasses operational challenges and limitations of the solid electrode where the oxygen is produced in the E-TAC technique by replacing it with NaBr aqueous electrolyte in water. This replacement paves the way for a continuous process (as opposed to a batch process with E-TAC) and repeals the need to swing cold and hot electrolytes alternately through the cell.
The bromide anions in the electrolyte are oxidized to bromate while producing hydrogen in a cathode, and they then flow with the aqueous electrolyte to a different cell, where they are turned back into their original state while at the same time producing oxygen, and this process keeps repeating itself.
In this way, hydrogen and oxygen are produced at the same time in two separate cells in a continuous process without any temperature changes, unlike with E-TAC. Moreover, the oxygen is produced in the aqueous electrolyte and not in the solid electrode as in E-TAC, and it is therefore not dependent on the rate and capacity limitations typical of those types of electrodes, such as chargeable batteries.
In the article published in Nature Materials, the researchers describe their basic experiments which prove the preliminary feasibility of the proposed process, and present results that demonstrate its high efficiency and ability to work at high electric current, meaning that hydrogen can be produced at a high rate. At the same time, there is still a long way ahead for developing a new technology based on the scientific breakthrough depicted in the article. Such a technology is likely to get past the many obstacles on the way to industrial production of green hydrogen as a sustainable alternative to fossil fuels.
More information: Ilya Slobodkin et al, Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte, Nature Materials (2024). DOI: 10.1038/s41563-023-01767-y