This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research lights up process for turning CO₂ into sustainable fuel

Researchers have successfully transformed CO2 into methanol by shining sunlight on single atoms of copper deposited on a light-activated material, a discovery that paves the way for creating new green fuels.
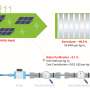
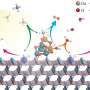
An international team of researchers from the University of Nottingham's School of Chemistry, University of Birmingham, University of Queensland, and University of Ulm have designed a material made up of copper anchored on nanocrystalline carbon nitride.
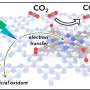
The copper atoms are nested within the nanocrystalline structure, which allows electrons to move from carbon nitride to CO2, an essential step in the production of methanol from CO2 under the influence of solar irradiation. The research has been published in the Sustainable Energy & Fuels journal.
In photocatalysis, light is shone on a semiconductor material that excites electrons, enabling them to travel through the material to react with CO2 and water, leading to a variety of useful products, including methanol, which is a green fuel. Despite recent progress, this process suffers from a lack of efficiency and selectivity.
Carbon dioxide is the greatest contributor to global warming. Although it is possible to convert CO2 to useful products, traditional thermal methods rely on hydrogen sourced from fossil fuels. It is important to develop alternative methods based on photo- and electrocatalysis, taking advantage of the sustainable solar energy and abundance of omnipresent water.
Dr. Madasamy Thangamuthu, a research fellow in the School of Chemistry, University of Nottingham, who co-led the research team, said, "There is a large variety of different materials used in photocatalysis. It is important that the photocatalyst absorbs light and separates charge carriers with high efficiency. In our approach, we control the material at the nanoscale. We developed a new form of carbon nitride with crystalline nanoscale domains that allow efficient interaction with light as well as sufficient charge separation."
The researchers devised a process of heating carbon nitride to the required degree of crystallinity, maximizing the functional properties of this material for photocatalysis. Using magnetron sputtering, they deposited atomic copper in a solventless process, allowing intimate contact between the semiconductor and metal atoms.
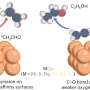
Tara LeMercier, a Ph.D. student who carried out the experimental work at the University of Nottingham School of Chemistry, said, "We measured the current generated by light and used it as a criterion to judge the quality of the catalyst. Even without copper, the new form of carbon nitride is 44 times more active than traditional carbon nitride."
"However, to our surprise, the addition of only 1 mg of copper per 1 g of carbon nitride quadrupled this efficiency. Most importantly, the selectivity changed from methane, another greenhouse gas, to methanol, a valuable green fuel."
Professor Andrei Khlobystov, School of Chemistry, University of Nottingham, said, "Carbon dioxide valorization holds the key for achieving the net-zero ambition of the UK. It is vitally important to ensure the sustainability of our catalyst materials for this important reaction. A big advantage of the new catalyst is that it consists of sustainable elements—carbon, nitrogen, and copper—all highly abundant on our planet."
This invention represents a significant step towards a deep understanding of photocatalytic materials in CO2conversion. It opens a pathway for creating highly selective and tunable catalysts where the desired product could be dialed up by controlling the catalyst at the nanoscale.
More information: Tara M LeMercier et al, Synergy of Nanocrystalline Carbon Nitride with Cu Single Atom Catalyst Leads to Selective Photocatalytic Reduction of CO2 to Methanol, Sustainable Energy & Fuels (2024). DOI: 10.1039/D4SE00028E