This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop high-energy, high-efficiency all-solid-state sodium-air battery
A research team has successfully developed a high-energy, high-efficiency all-solid-state sodium-air battery. This battery can reversibly utilize sodium (Na) and air without requiring special equipment. The team was led by Professor Byoungwoo Kang and Dr. Heetaek Park from the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH).
The findings have been published in the journal Nature Communications.
Secondary batteries find extensive use in green technologies such as electric vehicles and energy storage systems. The next-generation high-capacity secondary batteries, termed "metal-air batteries," draw power from abundant resources like oxygen and metals found on Earth. However, a challenge arises from the formation of carbonate—a byproduct of metal and oxygen reaction involving atmospheric carbon dioxide (CO2) and water vapor (H2O)—which sacrifices battery efficiency.
To address this, despite the name, metal-air batteries typically require additional equipment such as an oxygen permeation membrane to either purify oxygen or selectively use atmospheric oxygen.
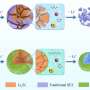
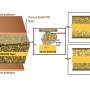
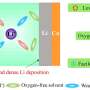
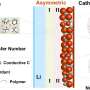
In this research, the team employed Nasicon, which is a Na superionic conductor and a solid electrolyte, to effectively tackle the carbonate issue. Nasicon, comprising elements like Na, silicon (Si), and zirconium (Zr), serves as a solid electrolyte capable of ion movement in the solid state while demonstrating high electrochemical and chemical stability.
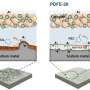
Leveraging this solid electrolyte, the team protected sodium metal electrodes from air and facilitated the breakdown of carbonate formed during electrochemical cell operation.
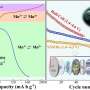
Consequently, the reversible electrochemical reaction involving carbonate led to an increase in the cell's energy density by increasing the working voltage while significantly reducing the voltage gap during charging and discharging, thus enhancing energy efficiency.
Moreover, the team's all-solid-state sodium-air cell exhibited superior kinetic capability through in-situ formed catholyte, which has a fast sodium ion conduction to the inside of the electrode. Remarkably, the cell operated solely on metal and air without additional special equipment for an additional oxygen filtration device.
Professor Kang who led the research remarked, "We've devised a method to harness carbonate, a longstanding challenge in the development of high-energy metal-air batteries. We hope to lead the field of the next generation all-solid-state metal-air batteries, leveraging a solid electrolyte-based cell platform that remains stable in ambient conditions and offers a broad voltage range."
More information: Heetaek Park et al, Activating reversible carbonate reactions in Nasicon solid electrolyte-based Na-air battery via in-situ formed catholyte, Nature Communications (2024). DOI: 10.1038/s41467-024-47415-0